Introduction
Risk management and risk assessment are critical components of operating a medical laboratory. These practices are essential not only for ensuring the safety of patients and laboratory personnel but also for maintaining compliance with regulatory standards. In the ever-evolving landscape of healthcare, where precision and accuracy are paramount, understanding and managing risks can significantly influence the quality of diagnostic outcomes.
Importance of Risk Management in Medical Laboratories
The Role of Risk Management in Ensuring Patient Safety
In medical laboratories, the stakes are high. Any error or mishap in the processing of samples, handling of chemicals, or operation of equipment can lead to incorrect diagnoses or delayed treatment, directly affecting patient outcomes. Risk management, therefore, plays a crucial role in identifying and mitigating these potential hazards to ensure that patients receive accurate and timely results.
Compliance with Regulatory Standards
Medical laboratories are subject to stringent regulations and standards, such as ISO 15189, which mandates a structured approach to risk management. Compliance with these regulations is not just a legal requirement but also a way to ensure that the laboratory’s operations are up to the mark, thereby fostering trust and credibility among patients and healthcare providers.
Share this article
Defining Risk Management and Risk Assessment
What is Risk Management?
Risk management in medical laboratories involves a systematic process of identifying, assessing, and controlling risks that could potentially affect the lab’s operations and outcomes. It encompasses everything from the strategic implementation of policies and procedures to the continuous monitoring and review of laboratory practices.
What is Risk Assessment?
Risk assessment is a subset of risk management, focusing specifically on evaluating the potential risks associated with specific activities, processes, or equipment. It involves identifying hazards, analyzing their potential impact, and determining the likelihood of their occurrence to prioritize which risks need the most attention.
The Framework of Risk Management in Medical Laboratories
Identifying Potential Risks
Biological Risks
Medical laboratories often handle a variety of biological samples, such as blood, tissues, and other bodily fluids, which may contain infectious agents. The risk of exposure to these pathogens poses a significant threat to lab personnel and requires stringent biohazard controls.
Chemical Risks
Chemicals used in testing and analysis can be hazardous if not handled correctly. Risks include exposure to toxic, corrosive, or flammable substances, which necessitates proper storage, labeling, and handling procedures.
Physical and Ergonomic Risks
Laboratory workers face physical risks from equipment such as centrifuges, autoclaves, and sharp instruments. Ergonomic risks, such as repetitive strain injuries from pipetting or working in awkward postures, are also prevalent in lab environments.
Risk Analysis
Qualitative vs. Quantitative Risk Analysis
Risk analysis can be approached qualitatively, where risks are described and categorized, or quantitatively, where risks are measured and expressed in numerical terms. Both methods are valuable in understanding the severity and probability of potential hazards.
Tools for Risk Analysis in Laboratories
Various tools can aid in risk analysis, including risk matrix where impact and probability are evaluated and root cause analysis. These methodologies help laboratories systematically evaluate and prioritize risks.
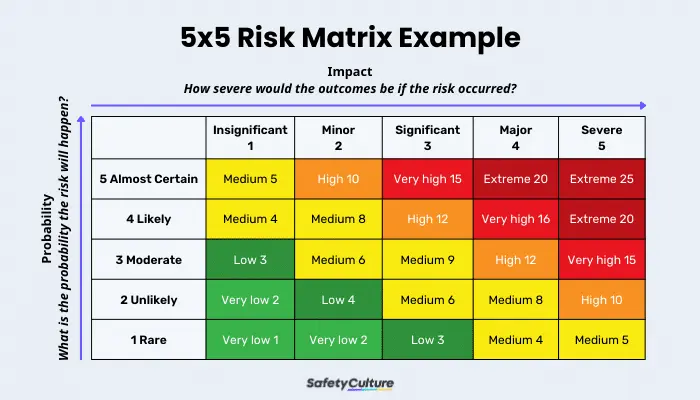
Risk Evaluation
Likelihood of Occurrence
Assessing the likelihood of a risk occurring is vital in prioritizing which risks require immediate attention. This involves reviewing past incidents, current controls, and the inherent nature of the laboratory’s work.
Impact Assessment
The impact of a risk refers to the potential consequences it could have on the laboratory’s operations, safety, and patient outcomes. High-impact risks, even if they have a low probability of occurrence, often need robust control measures.
Implementing Risk Control Measures
Hierarchy of Controls
Elimination and Substitution
The most effective way to control risks is to eliminate the hazard altogether or substitute it with a less dangerous alternative. For example, using automated systems to handle dangerous chemicals can reduce the need for direct human involvement.
Engineering Controls
When elimination or substitution isn’t possible, engineering controls, such as fume hoods, biological safety cabinets, and proper ventilation systems, can minimize exposure to hazards.
Administrative Controls
Administrative controls involve implementing procedures and policies to reduce risks. This includes developing standard operating procedures (SOPs), conducting regular training sessions, and ensuring compliance with safety protocols.
Personal Protective Equipment (PPE)
When other controls aren’t sufficient to mitigate risks, PPE such as gloves, lab coats, face shields, and respiratory protection must be used as a last line of defense.
Developing and Implementing Policies and Procedures
Standard Operating Procedures (SOPs)
SOPs are critical in ensuring consistent and safe laboratory operations. They provide step-by-step instructions for performing tasks and handling equipment, which helps in minimizing errors and reducing risks.
Training and Competency Assessment
Continuous training and competency assessments are essential in maintaining a high standard of safety and quality in the laboratory. These programs ensure that all personnel are aware of the risks and are capable of following established procedures.
Monitoring and Reviewing Risk Management Practices
Continuous Monitoring
Internal Audits
Regular internal audits help in identifying any lapses in risk management practices. These audits assess compliance with SOPs, the effectiveness of control measures, and the overall safety culture in the laboratory.
External Audits and Accreditation
Accreditation bodies, such as ISO, require external audits to ensure that laboratories meet specific standards. These audits provide an objective assessment of the laboratory’s risk management practices and identify areas for improvement.
Incident Reporting and Investigation
Root Cause Analysis
When an incident occurs, it’s crucial to conduct a thorough investigation to determine the root cause. This helps in understanding what went wrong and how similar incidents can be prevented in the future.
Corrective and Preventive Actions (CAPA)
CAPA is a key component of risk management, focusing on addressing the immediate issues (corrective actions) and implementing long-term solutions to prevent recurrence (preventive actions).
Challenges in Risk Management and Risk Assessment
Resource Constraints
Many laboratories, especially smaller ones, may face resource constraints that limit their ability to implement comprehensive risk management practices. This includes financial limitations, staffing shortages, and lack of access to advanced technology.
Human Factors and Errors
Human error is a significant challenge in risk management. Despite the best controls and training, mistakes can happen, and these can have serious consequences in a laboratory setting.
Technology and Data Management Issues
As laboratories become more reliant on technology, issues related to data management, cybersecurity, and equipment failures become more prevalent. These technological risks need to be addressed as part of a holistic risk management strategy.
The Future of Risk Management in Medical Laboratories
Role of Automation and AI
Automation and AI are transforming risk management in medical laboratories. These technologies can reduce human error, enhance accuracy, and provide real-time monitoring of laboratory conditions, making risk management more efficient.
Integration with Quality Management Systems (QMS)
Integrating risk management with QMS allows for a more streamlined approach to managing laboratory operations. This integration ensures that risk management practices are aligned with the laboratory’s overall quality objectives.
Evolving Regulatory Requirements
As regulations evolve, laboratories must adapt their risk management practices to remain compliant. This requires staying updated with the latest standards and ensuring that risk management frameworks are flexible enough to accommodate changes.
Conclusion
Risk management and risk assessment are indispensable for ensuring the safety, accuracy, and efficiency of medical laboratories. By implementing a robust risk management framework, laboratories can minimize potential hazards, comply with regulatory standards, and ultimately contribute to better patient outcomes. As the field of healthcare continues to evolve, so too must the practices and tools used to manage risks, ensuring that medical laboratories remain safe and reliable environments.
FAQs
What is the primary goal of risk management in medical laboratories?
The primary goal of risk management in medical laboratories is to identify, assess, and mitigate potential risks that could affect the safety of patients, staff, and the accuracy of test results.
How often should risk assessments be conducted in a laboratory?
Risk assessments should be conducted regularly, at least annually, or whenever there are significant changes in laboratory processes, equipment, or personnel.
What is the difference between risk assessment and risk management?
Risk assessment is the process of identifying and evaluating potential risks, while risk management involves implementing strategies to control and mitigate those risks.
Can automation completely eliminate risks in medical laboratories?
While automation can significantly reduce certain risks, it cannot completely eliminate all risks, especially those related to human factors, equipment failures, and unforeseen circumstances.
How can small laboratories manage risk with limited resources?
Small laboratories can manage risk by prioritizing high-impact risks, using cost-effective control measures, leveraging external resources like accreditation bodies, and ensuring continuous training and competency of their staff.


