Biosafety Levels in Clinical Labs: What You Need to Know
Biosafety Levels in Clinical Labs: What You Need to Know
Introduction
Biosafety is a crucial aspect of laboratory operations, ensuring that laboratory personnel, the public, and the environment are protected from potentially hazardous biological agents. In clinical labs, biosafety measures are categorized into four distinct levels, each with specific containment protocols and safety requirements. This article will delve into the details of these biosafety levels (BSLs), their importance, and the practices associated with each level.
What Are Biosafety Levels?
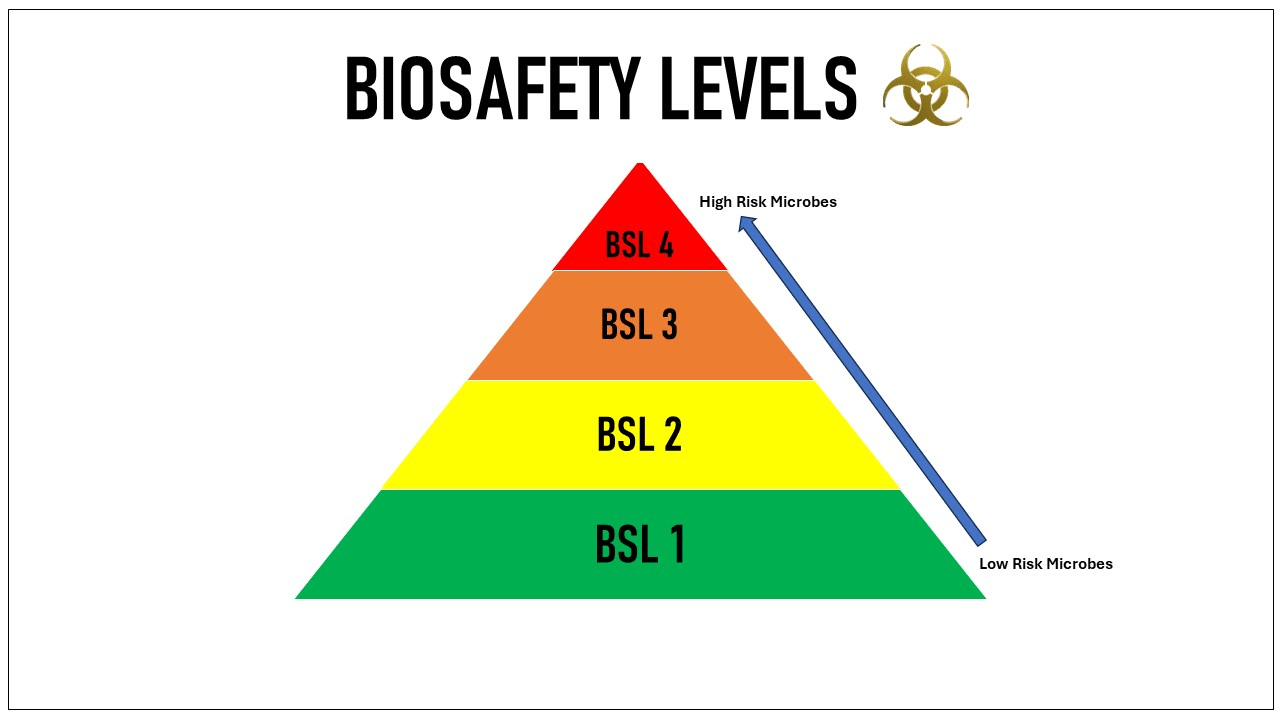
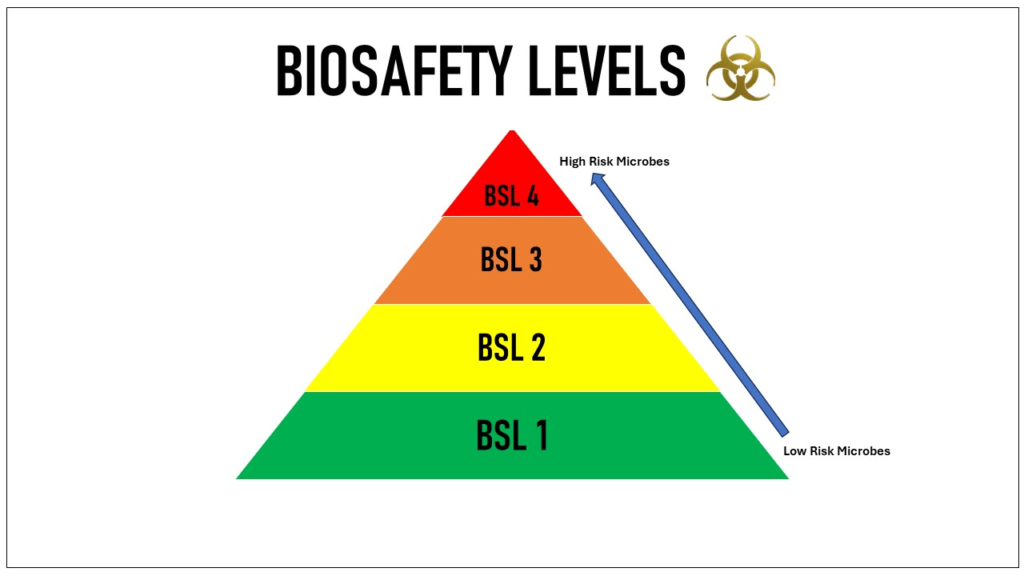
Biosafety levels are a set of biocontainment precautions required to isolate dangerous biological agents in an enclosed laboratory facility. The levels range from BSL-1, which requires the least containment, to BSL-4, which requires the highest level of containment. The Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) define these levels based on the risk posed by the pathogens studied and the laboratory practices needed to safely handle them.
Overview of Biosafety Levels
Biosafety Level 1 (BSL-1)
Description:
BSL-1 is the basic level of biosafety and applies to laboratories that work with agents not known to consistently cause disease in healthy adults. These labs typically handle non-pathogenic strains of bacteria and viruses, posing minimal potential threat to laboratory personnel and the environment.
Key Practices:
- Standard microbiological practices are followed.
- No special primary or secondary barriers are required other than a sink for handwashing.
- Personal protective equipment (PPE) such as lab coats and gloves are recommended.
- Access to the lab is limited when work is being conducted.
Examples of Agents:
- Non-pathogenic Escherichia coli (E. coli)
- Bacillus subtilis
Biosafety Level 2 (BSL-2)
Description:
BSL-2 is suitable for work involving agents that pose moderate hazards to personnel and the environment. These agents can cause human disease of varying severity.
Key Practices:
- All BSL-1 practices are followed.
- Laboratory personnel have specific training in handling pathogenic agents.
- Access to the lab is restricted.
- Procedures that generate infectious aerosols or splashes are conducted in a biological safety cabinet (BSC).
- An autoclave is available for decontaminating waste.
- PPE includes lab coats, gloves, face protection, and possibly face shields.
Examples of Agents:
- Hepatitis B virus
- HIV
- Staphylococcus aureus
Biosafety Level 3 (BSL-3)
Description:
BSL-3 labs handle indigenous or exotic agents that may cause serious or potentially lethal disease through the inhalation route. The emphasis is on containment and environmental controls.
Key Practices:
- All BSL-2 practices are followed.
- Laboratory personnel undergo medical surveillance and may receive immunizations for agents they work with.
- Laboratory access is strictly controlled and monitored.
- All work is performed within a BSC or other containment devices.
- The laboratory is designed with directional airflow to prevent contamination.
- Respiratory protection may be required.
Examples of Agents:
- Mycobacterium tuberculosis
- West Nile virus
- Yellow fever virus
Biosafety Level 4 (BSL-4)
Description:
BSL-4 is required for work with dangerous and exotic agents that pose a high risk of aerosol-transmitted infections and life-threatening disease. There are no available vaccines or treatments for these agents.
Key Practices:
- All BSL-3 practices are followed.
- Laboratory personnel must change clothing before entering and shower upon exiting.
- All materials are decontaminated before leaving the facility.
- Work is performed in a Class III BSC or by personnel wearing a full-body, air-supplied, positive pressure suit.
- The laboratory is located in a separate building or a completely isolated and restricted zone.
Examples of Agents:
- Ebola virus
- Marburg virus
Importance of Biosafety Levels
Understanding and implementing appropriate biosafety levels in clinical labs is critical for several reasons:
- Protecting Laboratory Personnel: Ensures the safety of lab workers by minimizing their exposure to hazardous agents.
- Preventing Environmental Contamination: Reduces the risk of accidental release of pathogens into the environment.
- Compliance with Regulations: Adheres to national and international biosafety standards and regulations.
- Maintaining Public Health: Prevents outbreaks of infectious diseases by containing potentially harmful biological agents.
Conclusion
Biosafety levels are essential for the safe and effective operation of clinical laboratories. Each level requires specific practices, equipment, and facility design to ensure the containment of biological agents and the safety of personnel and the public. By adhering to these biosafety guidelines, laboratories can conduct research and diagnostics without compromising safety.
For further information on biosafety levels, refer to resources from the CDC and WHO, which provide comprehensive guidelines and protocols for laboratory safety.
References
- Centers for Disease Control and Prevention (CDC). (2020). Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th Edition.
- World Health Organization (WHO). Laboratory Biosafety Manual, 3rd Edition.
- Public Health Emergency (PHE). Biosafety Levels.
- OnePointe Solutions. Biosafety Levels Explained for 2020.
- Consteril. Biosafety Levels 1, 2, 3 & 4: What’s the Difference?
By adhering to the outlined biosafety levels, clinical laboratories can ensure a high standard of safety and compliance, ultimately contributing to better public health outcomes.